The clinical trial is closed
however, this information appears for those patients who are participating in the clinical trial
Motiva breast implants are considered an investigational device in the US and Canada and subjects will be participating in an FDA approved clinical trial.
What is a Breast Implant Clinical Trial?
A clinical trial is a research study that allows manufacturers to evaluate the safety and efficiency of their new product. In order to get a new breast implant onto the market, manufacturers must first enroll subjects in clinical trials across the country as an investigational study. Although the study is investigational, the implants are not experimental. Many implants in clinical trials are approved in other countries and often have been in use for years prior to their US debut.
How do I Take Part in the Clinical trial?
Each applicant will be reviewed based on the specific inclusion and exclusion criteria to determine if they are a candidate for the lengthy trial period. You will still need to come into our office and sit down for a consultation prior to the surgery. Dr. Epstein will discuss the Motiva Implants with you to determine size and shape/implant type.
Am I a Candidate for the Motiva Implant Clinical Trial?
The Inclusion Criteria is as Follows:
- Genetic female.
- Patient is seeking one of the following procedures: Primary Breast Augmentation: age 22 or over, indicated to increase breast size. Primary Breast Reconstruction: to replace breast tissue that has been removed due to cancer, prophylactic mastectomy, breast trauma or that has failed to develop properly due to a severe breast anomaly. Breast Implant Revision Surgery: (removal and replacement of breast implants) revision surgery to correct or improve the results of a previous breast augmentation or reconstruction surgery.
- Patient has adequate tissue available to cover implant(s).
- Willingness to follow all study requirements including agreeing to attend all required follow-up visits and signs the informed consent.
- Agrees to have device returned to Establishment Labs, if explanted.
- Willing to undergo Magnetic Resonance Imaging (MRI) evaluation if medically advised.
Exclusion Criteria is as Follows:
- Has any breast disease considered to be pre-malignant, or an untreated cancer of any type without mastectomy or has medical history reporting mutations in BRCA1 or BRCA2 of both breasts or one breast.
- Has inadequate or unsuitable tissue (e.g., due to radiation damage, ulceration, compromised vascularity, history of compromised wound healing).
- Has an abscess or infection.
- Is pregnant or nursing or has had a full-term pregnancy or lactated within 6 months of enrollment.
- Is taking any drugs that would interfere with blood clotting, or that might result in elevated risk and/or significant postoperative complications.
- Has any medical condition such as obesity (BMI >40), diabetes, autoimmune disease, chronic lung or severe cardiovascular disease that might result in unduly high surgical risk and/or significant postoperative complications.
- Has any connective tissue/autoimmune disorder or rheumatoid disease, such as systemic lupus erythematosus, discoid lupus, scleroderma, or rheumatoid arthritis, among others.
- Has any condition that impedes use of magnetic resonance imaging (MRI) including implanted metal device, claustrophobia or other conditions that would make MRI scan prohibited.
- Has a history of psychological characteristics that are unrealistic or unreasonable given the risks involved with the surgical procedure.
- Has been implanted with any non-FDA approved breast implant.
- Has been implanted with any silicone implant other than breast implants.
- HIV positive (based on medical history).
- Has been diagnosed with anaplastic large cell lymphoma (ALCL).
- Works for Establishment Labs, Motiva USA or any of their subsidiaries, the study surgeon, or ICON the Contract Research Organization (CRO) that is helping to conduct the study, or are directly-related to anyone that works for Establishment Labs, Motiva USA or any of their subsidiaries, the study surgeon, or the CRO.
Will I be Receiving Free Surgery?
The clinical trial is closed
however, this information appears for those patients who are participating in the clinical trial
Although implants are often part of the surgery cost, Motiva Implants are provided free of charge at the day of surgery. You will be responsible for the cost of the surgery and any routine surgical appointments following. You will also receive compensation for being part of the trial which will be awarded after each annual visit. Visit our Patient Information page to learn more.
Does this Clinical Trial Consist of Only One Surgery?
As a participant in the Motiva clinical trial, you will undergo breast augmentation surgery with Dr. Epstein in Hauppauge, Long Island. By participating in the trial, you are committing to coming back each year to visit with Dr. Epstein for the course of 10 years. This does not require additional surgeries but could involve additional testing including MRIs. Additional surgeries may be required if there are complications or if a patient desires to go bigger or smaller in volume.
Are There Any Risks Specific to Motiva Silicone Gel Breast Implants?
- RFID microtransponder Q Inside Safety Technology – The implant may or may not contain a transponder with a radiofrequency identification device (RFID) for traceability purposes. Risks identified for the microtransponder will be addressed by compliance with special controls established for the device. In rare cases, a suspicion of microchip failure could be identified because no electronic serial number is obtained in the reading with the Motiva Q inside Reader Standard, in this case the site investigators will communicate the sponsor to further analyze and verify the reason of the suspected failure.
- Blue pigment – The barrier layer of the Motiva implants includes a blue silicone pigment to enable visual recognition of this layer by surgeons and quality inspectors. The pigment content represents less than 0.05% of the barrier layer material and less than 0.008% of all shell layers.
- Unknown Risks- Concerns include immunological and neurological disorders, carcinogenicity and connective tissue disorders due to the relationship between silicone and certain diseases
- Connective Tissue Disease, Cancer, Neurological Disease
To learn more about the Motiva Implants® clinical trial in the United States from Establishment Labs, click here.
Motiva Clinical Trial Patient Information
How Much Will My Breast Augmentation Cost Under the Clinical Trial?
By participating in the Motiva Implant Clinical Trial, you are agreeing to receive free breast implants the day of surgery with follow up visits for the following 10 years. You will still be responsible for covering the cost of the surgery as discussed with your surgeon. Contact our office today to learn more and schedule your consultation.
Patient Compensation After Surgery
The clinical trial is closed
however, this information appears for those patients who are participating in the clinical trial
As a participant in this clinical trial, you are agreeing to annual follow up visits for 10 years after surgery. You will be compensated with a Cash Bonus and Trust Fund which varies based upon your procedure. Patients who are receiving Primary Breast Augmentation and Revision Augmentation are in Group 1, while patients who are receiving Primary Breast Reconstruction and Revision Reconstruction are in Group 2.
Group 1: Compensation for Patients Undergoing Primary Breast Augmentation and Revision Augmentation Surgery
Patients participating in the Study will receive the following compensation:
- Paid consultations for all follow-up visits with their treating study doctors, to evaluate the Motiva Implants ™.
- A cash bonus as described in the table below, to be paid to the Patient after every follow-up visit:
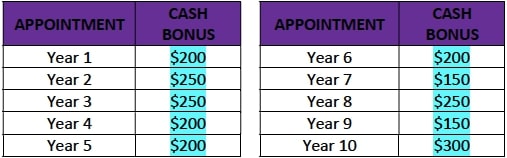
- After the first annual follow up visit (Year 1), Motiva USA will deposit into the trust fund the full amount of $750, corresponding to the total of all trust fund installments, according to the table below. With every annual follow up visit the patient will vest the corresponding amount.
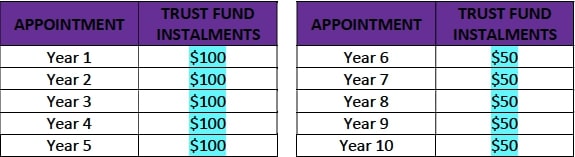
- If a patient drops out of the Study before completion, the total $750 corresponding to her in the trust fund will be pooled and divided equally among the patients that finish the study. Additionally, any patient who misses two consecutive annual visits will be considered lost to follow up and will only receive the cash payment for the study visits attended and keep the implants. Only patients that complete the 10-year follow-up will receive the total of all trust fund installments.
- Both, cash bonuses and the total of the Trust Fund Installments, will be paid directly to the patient.
- If a patient misses a follow-up visit and/or drops out of the Study, she will not receive the remaining cash bonuses or the total of the Trust Fund Installments but will keep the breast implants at no charge.
Group 2: Compensation for Patients Undergoing Primary Breast Reconstruction and Revision Reconstruction Surgery
Patients participating in the Study undergoing Primary breast reconstruction and revision
surgery will receive the following compensation:
- Paid consultations for all follow-up visits with their treating study doctors, to evaluate the Motiva Implants ™.
- A cash bonus as described in the table below, to be paid to the Patient after every follow-up visit:
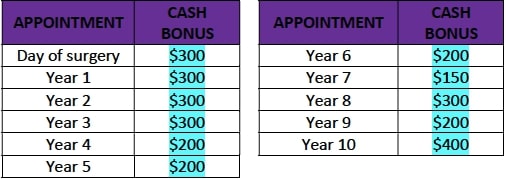
- After the first annual follow up visit (Year 1), Motiva USA will deposit into the trust fund the full amount of $950, corresponding to the total of all trust fund installments, according to the table below. With every annual follow up visit the patient will vest the corresponding amount.
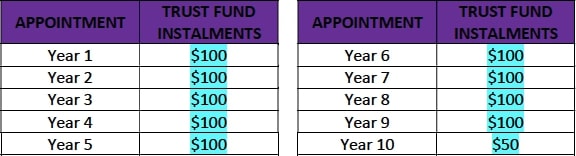
- After the Study is finalized and the patient has attended all follow up visits, she will receive the total of all Trust Fund Installments.
- If a patient drops out of the Study before completion, the total $950 corresponding to her in the trust fund will be pooled and divided equally among the patients that finish the study. Additionally, any patient who misses two consecutive annual visits will be considered lost to follow up and will only receive the cash payment for the study visits attended and keep the implants. Only patients that complete the 10-year follow-up will receive the total of all trust fund installments.
- Both, cash bonuses and the total of the Trust Fund Installments, will be paid directly to the patient.
- If a patient misses a follow-up visit and/or drops out of the Study, she will not receive the remaining cash bonuses or the total of the Trust Fund Installments but will keep the breast implants at no charge.
Schedule for Patient Visits Following Surgery
The clinical trial is closed
however, this information appears for those patients who are participating in the clinical trial
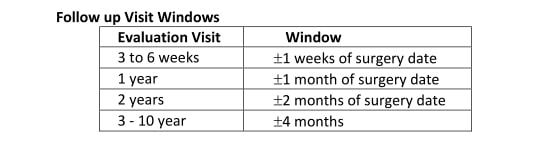
Following surgery, patients will return to our office for follow up visits for 10 years. The schedule for these visits is as follows:
MRIs After Surgery
The FDA recommends that all patients should have regular MRI screenings throughout their lifetime to screen for silent rupture, even if they are not having any apparent problems. Patients should go for their first MRI 3 years after surgery, and then repeatedly every 2 years following.
MRI Sub-Study
As part of the Motiva Clinical Trial, 250 patients will be divided into 7 MRI sites to participate in an MRI Sub-Study. The MRI Sub-Study population will be a subset of the treated population selected to obtain MRIs at 1, 2, 4, 6, 8, and 10 years. It will be used for the analysis of rupture. Motiva USA will cover the cost of the patients MRIs. Any patient not chosen as part of the Sub-Study will be responsible for the cost of their own MRIs.
Sections - Motiva Breast Implants
- What is the Motiva Breast Implant?
- Ergonomix vs Round: Breast Shape and Movement
- Dr. Epstein’s Seven Year Motiva Experience
- What Makes the Motiva Implant Different?
- Motiva – Is it Smooth or Textured?
- Motiva vs Other Implants
- How Motiva Implants Are Different from Allergan Natrelle, Mentor and Sientra Implants
- MOTIVA US Clinical FDA Trial
Prev Chapter: Intro to Breast Augmentation »
Next Chapter: Five key decisions you need to make »
Chapters – Breast Augmentation Guide
- Intro to Breast Augmentation
- Motiva Breast Implants
- Five key decisions you need to make
- One-Day Recovery Breast Augmentation
- Anesthesia – General, Sedation or Local?
- Breast Lift (Mastopexy) with/without Implants or Fat
- What else should I know about breast augmentation?
- Important Things to Consider When You Decide to Move Forward With Breast Augmentation
- Revision of breast augmentation
- ALCL and Breast Implant Illness