Why Do We Care if a Breast Implant Has a Textured Surface?
The concern about the whether or not a breast implant has a textured surface is because textured surfaces have been associated with a rare malignancy arising in the fibrous capsule surrounding the breast implant, NOT the breast tissue itself. This is called Anaplastic Large Cell Lymphoma (ALCL). This is a curable disease and there are less than 1000 such cases worldwide, as compared to the many millions of women who have undergone breast implant surgery. Among the different manufacturers of textured breast implants, it appears that the frequency of ALCL is higher in implants with a greater degree of texturing. What is texturing? Think of it like an english muffin with "nooks and crannies." The greater the height of the peaks of the nooks and crannies, the greater the degree of the texturing. These peaks can actually be measured, along with other parameters describing the surface topology of the implant.
The significance of all this is that the body's immune system sees the breast implant *or any foreign object at all) as a foreign body and attempts to "wall it off", to protect the body from the foreign invader. This is not rejection as seen with an organ transplant, but more of a protective bio-mechanical response. Various cells such as fibroblasts and macrophages recognize the breast implant as foreign. What these cells are interacting with is the implant's surface.
Different surface topologies can either disguise or expose the implant from the body's defense system, somewhat analogous to the way a F14 fighter jet might appear visible on enemy defense radar but a B2 stealth bomber would not. There is just something about the surface that either magically screams "I am a breast implant and I am here" or hides the implant under "cloak of darkness."
What is the Short Answer?
For those of you who just want a quick answer, ALL MOTIVA IMPLANTS ARE NOT TEXTURED. They are labelled by the FDA as a "SilkSurface" device.
What is the Long Answer?
Is Motiva a Textured Device?
The long answer is a bit more complicated. Only microtextured (Mentor Siltex, Sientra, Allergan Microcell) and macrotextured implants (Allergan Biocell) are associated with Anaplastic Large Cell Lymphoma (ALCL). The Allergan Biocell implant, most likely to their greater degree of texturing, have the highest association with ALCL. It appears that the degree of texturing correlates with the incidence of ALCL.
Smooth implants are not associated with ALCL. Motiva implants are classified by the FDA as a "4 micron SilkSurface device." There are no known cases of ALCL arising in smooth implants nor Motiva SilkSurface implants.
The Motiva implant has a 4 micron SmoothSilk surface which affords it some rather unique properties. As compared to traditional smooth as well as to microtextured and macrotextured implants, Motiva implants have been shown to have a lower incidence of bacterial adherence and inflammatory cell (fibroblast and macrophage) adherence to its surface. There is insignificant particulate shedding due to implant wear. There is less inflammatory cell mediation. All this translates to a lower amount of inflammation and tissue reaction between Motiva implants and the body, leading to thinner capsules (the normal scar tissue that forms around ALL breast implants and other implanted medical devices), and a lower rate of capsule contracture in published studies.
When compared to other breast implants, Motiva safety data demonstrates a lower rate of capsule contracture, implant rupture and other device related complications. This translates to lower re-operation rates, associated costs and inconveniences.
Watch this video to better understand why the Motiva SmoothSilk surface is superior to other breast implant surfaces.
There is actually a classifcation scheme for texturing of surfaces in breast implants. The 2018 ISO (International Organization for Standardization) classified implant surfaces as follows:
- Smooth: < 10 Microns of roughness:
- Microtextured: 10 - 50 Microns of roughness
- Macrotextured: > 50 Microns of roughness
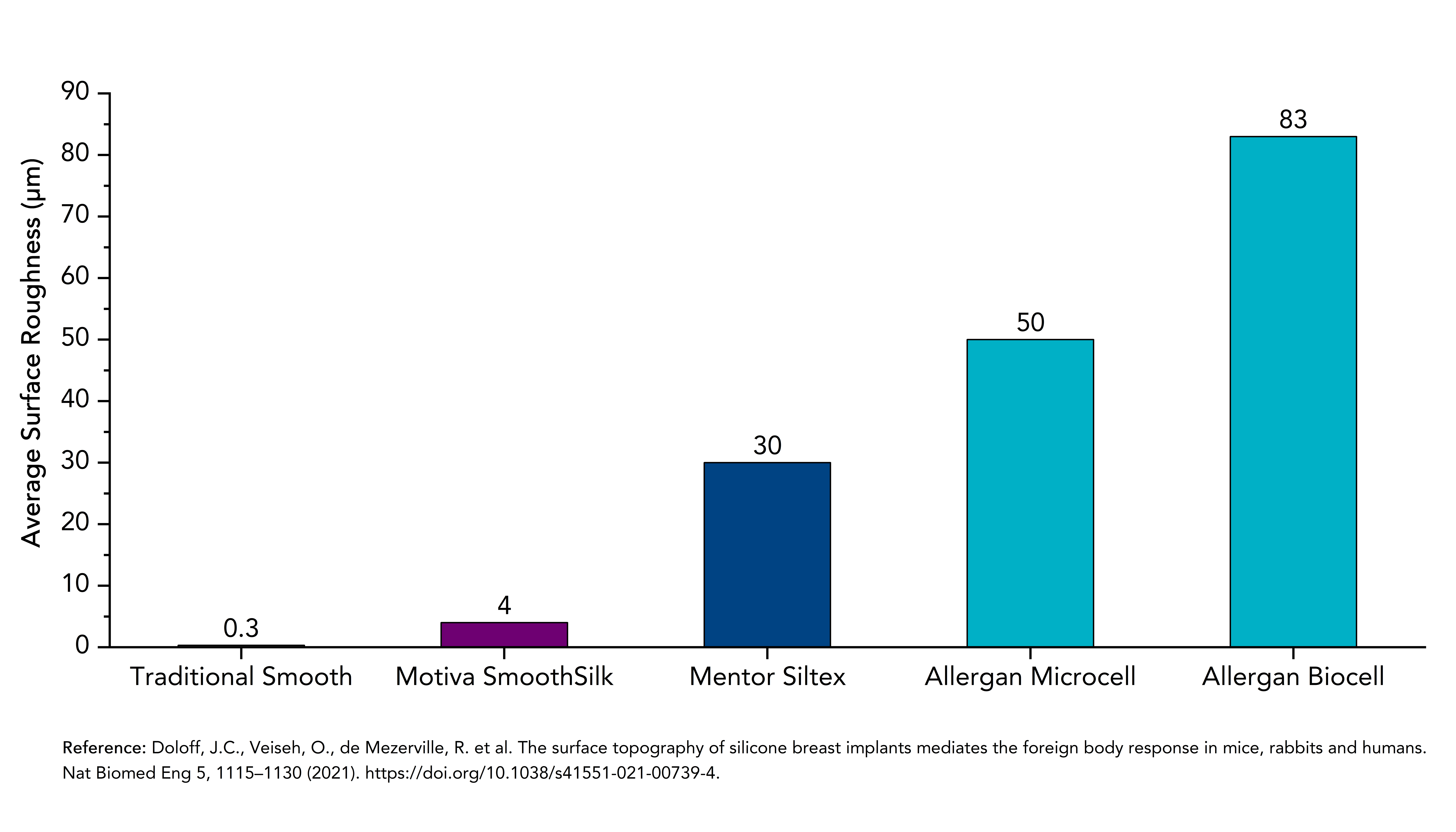
Several common breast implants from various manufacturers are shown in the chart below
- Smooth category: are the traditional smooth implants (0.3 microns - which as previously stated are really not smooth) and the Establishment Labs Motiva SilkSurface/SmoothSIlk surface technology (4 microns). SilkSurface and SmoothSIlk are really two different terms used interchangeably to refer to the same surface technology.
- Microtextured category: Mentor Siltex (30 microns) and Sientra's (40 microns) (not shown) surface technology.
- Macrotextured category: Allergan Natrelle Microcell (50 microns) and Biocell (83 microns). The Biocell implants were recalled in 2019.
You can see that the Allergan Natrelle Teardrop implant (Biocell surface) has the most texturing. It did probably the best job of holding the implant in place, but also had the highest rate of ALCL. The theory behind this was probably that certain species of bacteria could hide from the bodies immune defenses more easily in the nooks and crannies (like an english muffin) of an implant surface with more roughness.
The ISO classification scheme was changed in 2024. The current scheme does not make a lot of sense, nor does it seem to follow what is already known in the published scientific literature. That said, for the purposes of discussion, we will assume that smooth surface implants are in fact smooth. Classification schemes are all semantics and it is important to not get led down a "rabbit-hole" by this. It is best to look at the individual mechanical and biological characteristics of a device rather than a "classification scheme" which can be very misleading.
Currently, Motiva devices are classified by the FDA and are labeled as such as a "SilkSurface" device. The word "texturing" does not appear anywhere on the package labelling. If you want to get technical, you could call Motiva SilkSurface a 3 micron textured surface, but if you do, then you should call a smooth surface a 0.3 micron textured surface. The bottom line is, you cannot consider a Motiva implant surface to be anything like a macrotextured surface like the Allergan Natrelle Biocell surface that was recalled for its relationship to ALCL. Similarly, you cannot consider the Motiva SIlkSurface to be anything like the Mentor and Sientra microtextured surfaces.
What really matters is not semantics (ie what the surface classification is) but rather the following three aspects of evaluation:
- In-vivo behaviour - how the surface interacts with the bodies tissues - inflammation, capsule formation and thickness
- Bench level testing - Experimental testing to see how living cells react to the surface
- Clinical outcome data - Capsule contracture rate, rupture rate, reoperation rate and other device related complication data
The Motiva SilkSurface exceeds all other implants in all three of these areas of evaluation.
Sections - Motiva Breast Implants
- What is the Motiva Breast Implant?
- Ergonomix vs Round: Breast Shape and Movement
- Dr. Epstein’s Seven Year Motiva Experience
- What Makes the Motiva Implant Different?
- Motiva – Is it Smooth or Textured?
- Motiva vs Other Implants
- How Motiva Implants Are Different from Allergan Natrelle, Mentor and Sientra Implants
- MOTIVA US Clinical FDA Trial
Prev Chapter: Preservation Breast Augmentation – A New Era! »
Next Chapter: Five key decisions you need to make »
Chapters - Breast Augmentation Guide
- Intro to Breast Augmentation
- Preservation Breast Augmentation – A New Era!
- Motiva Implants – What You Need to Know
- Five key decisions you need to make
- One-Day Recovery Breast Augmentation
- Anesthesia – General, Sedation or Local?
- Breast Lift (Mastopexy) with/without Implants or Fat
- What else should I know about breast augmentation?
- Important Things to Consider When You Decide to Move Forward With Breast Augmentation
- Revision of breast augmentation
- ALCL and Breast Implant Illness